The researchers then engineered human pluripotent stem cells -- cells that have the potential to develop into any cell type -- to make them more amenable to integration and less likely to self-destruct by temporarily shutting down apoptosis.
Then, they converted these cells into "naive" cells resembling early human embryonic cells by culturing them in a special medium.
Before implanting the developing embryos in surrogate sows, the researchers grew the chimeras in conditions that were optimised to provide unique nutrients and signals to both the human and pig cells, since these cells usually have disparate needs.
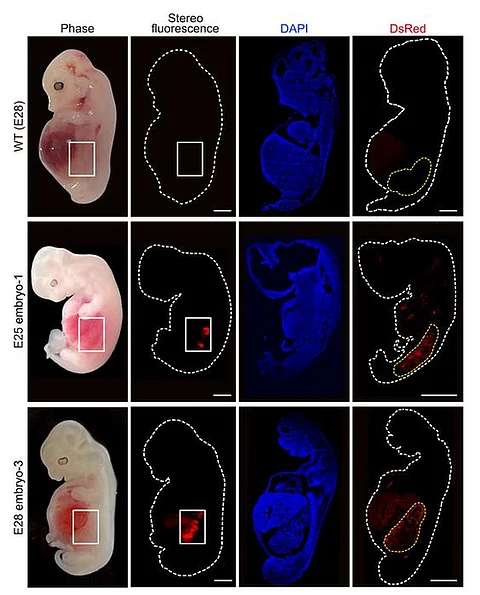
Altogether, the researchers transferred 1,820 embryos to 13 surrogate mothers. After either 25 or 28 days, they terminated gestation and extracted the embryos to assess whether the chimeras had successfully produced humanised kidneys.
The researchers collected five chimeric embryos for analysis (two at 25 days and three at 28 days post-implantation) and found that they had structurally normal kidneys for their stage of development and were composed of 50-60 per cent human cells.